Levin, L.A., Marine Life Research Group, Scripps Institution
of Oceanography, La Jolla, CA 92093-0218, USA llevin@ucsd.edu
Diaz, R.J. Virginia Institute of Marine Science, College of William and
Mary, Gloucester Pt., VA 23062-1346, USA, diaz@vims.edu
MACROFAUNAL COMMUNITY RESPONSE TO HYPOXIA: A COMPARISON OF PERMANENT VERSUS EPISODIC EXPOSURE
Large areas of the bathyal seafloor within oxygen minimum zones and some
fjords and basins experience permanent severe hypoxia (<0.5 ml O2 per
l). Macrofaunal communities within these regions exhibit reduced (or enhanced)
densities, low species richness and evenness, and high dominance by annelids.
Dominant species exhibit varying lifestyles and nutritional modes. These
assemblages differ from shallower communities exposed to seasonal or episodic
hypoxia in having: (a) much lower oxygen tolerance thresholds, (b) morphological
adaptations to maximize respiratory surface, (c) specialist rather than
opportunistic lifestyles, and (d) potential to utilize chemosynthesis-based
nutritional pathways. Similarities between the systems include reduced
macrofaunal diversity, commonality of family level taxa such as spionid
polychaetes, tubificid oligochaetes and ampeliscid amphipods. Temporal
and spatial stability of dissolved oxygen concentration appears to be
a primary factor regulating community structure and function in both OMZs
and shallow coastal hypoxic areas. Comparisons of the two regimes should
contribute to better understanding of changes to be expected as hypoxic
conditions increase in spatial and temporal extent.
ASLO MEETING Feb. 2001
Oxygen Minimum Zone Influence on the Community Structure of Deep-Sea Benthos.
Lisa A. Levin, Marine Life Research Group, Scripps Institution of Oceanography, La Jolla, CA 92093-0218 USA
Introduction
Oxygen minimum zones (OMZs) are midwater regions of the ocean with hypoxic waters, where oxygen concentrations typically are < 0.5 ml L-1 (or about < 20mM). They usually occur in the middle of the water column at upper bathyal depths (200-1200 m) (Wyrtke 1971, 1973). OMZs generally form where strong upwelling leads to high surface productivity that sinks and degrades, depleting oxygen within the water column. OMZ formation is most intense in regions of sluggish circulation and where there are source waters already low in oxygen. OMZs occur in much of the eastern Pacific Ocean, in the Arabian Sea and off W. Africa (Kamykowski and Zentara 1991). Deep-water hypoxia also is found in deep basins in the southern California borderland and in some fjords (Diaz and Rosenberg 1995)..
A typical vertical profile of oxygen concentration through an OMZ exhibits a steep drop in oxygen at upper boundary, within the top 100-200 m. Below this there is a zone of continuous low oxygen, with concentrations often << 0.1 ml/l. The lower OMZ boundary exhibits a gradual increase in oxygen with water depth. The thickness of the OMZ varies regionally, and is strongly influenced by circulation. Off Mexico and in the Arabian Sea the OMZ is about 1000 m thick (Wyrtki, 1973), but off Peru, Chile and California, the OMZ is only few hundred m thick (Wyrtki, 1966).
OMZs differ from many shallow-water hypoxic regions in exhibiting stable, persistent low oxygen over ecological time scales, such that sessile species will live out many generations in continuous low oxygen. At the upper OMZ boundary, oxygen concentrations can vary with internal tides (Levin et al.,1991) or larger-scale oceanographic forcing like El Nino (Tarazona et al. 1988), but this variation usually has little effect on core values. OMZ intensity and distribution does vary on geological time scales. Shifts in productivity or circulation over thousands of years are thought to drive expansion and contractions of OMZ both vertically and horizontally (Tyson and Pearson 1991, Rogers, 2000).
Where oxygen minimum zones impinge on the seafloor they create strong gradients in bottom- water oxygen concentration. Associated with these low oxygen values are high organic carbon contents of sediments. POC values of 4-6% are typical of many OMZs (Levin and Gage, 1998), but off Peru they can reach 16% (Levin et al. in preparation). There is generally an inverse relationship between bottom-water oxygen concentration and sediment POC in bathyal sediments.
Another feature of many OMZs is cover by large, filamentous, sulfur oxidizing, nitrate reducing bacteria (Jorgensen and Gallardo, 1999). These are typically Thioploca, but sometimes include Beggiatoa, and may form mats, tufts or a thin, grass-like cover (Levin, pers. observation)
Benthic Response to OMZ Conditions
Many aspects of benthic ecosystems vary within oxygen minimum zones. Aspects of community structure including size, abundance, taxonomic composition, diversity and lifestyles, are distinct within sediments intercepted by OMZ. Ecosystem functions such as bioturbation and trophic pathways also vary within versus beneath OMZs.
Perhaps the most inclusive benthic system response to OMZ conditions is altered size structure. At very low oxygen levels (<0.1ml/l) the fauna often consists of meiofaunal size organisms (protozoans and metazoans); macrofauna and megafauna are typically rare or absent (Levin et al. 1991, pers. obs). This is true in the Santa Barbara Basin (Bernhard et al. 2000), and on a Volcano 7, a seamount off Mexico, where the summit protrudes into the eastern Pacific OMZ (Levin et al. 1991). On Volcano 7, O2 increases downslope in a linear fashion. Bacteria are abundant at the summit, where oxygen is lowest but little else occurs there except nematodes. The macrofauna are rare; megafauna are absent or sparse (Levin et al. 1991, Wishner et al. 1995). Presumably small-bodied animals have an advantage in severe hypoxia, by having a larger surface area-volume ratio. Multiple regressions were employed to examine relationships between environmental factors and densities of bacteria, meiofauna and macrofauna. These suggest that densities of the bacteria and metazoan meiofauna are related largely to measures of organic matter availability (e.g., Chlorophyll a, POC, PON. Bottom-water oxygen concentration was correlated only with macrofaunal densities. Megafaunal densities were not tested.
Distinct abundance trends are seen within OMZs for some taxa but not others. Total densities of meiofauna are never reduced within OMZs, and often reach maximum bathyal values within OMZs, presumably due to abundant particulate food and/or reduced predation intensity (Neira et al., in press). In contrast, abundances of macrofauna are sometimes depressed (but not always). They often exhibit a maximum or peak where oxygen levels climb just slightly,to concentrations of 0.1 –0.2 m/l. This pattern has been observed off central California, West Africa, off Mexico (Volcano 7) and on the Oman margin in the Arabian Sea (Fig.1). It has sometimes been called a boundary effect, but often these maxima occur well above the OMZ lower boundary (technically defined as 0.5 ml/l). Some sort of physiological threshold appears to create this pattern. Once oxygen rises sufficiently, a small number of tolerant species are able to attain exceptionally high densities due to the great food supply. We have not observed this effect off Peru (Levin et al, in preparation). Similar thresholds occur in the megafauna as illustrated by counts on Volcano 7 (Wishner et al. 1990, 1995). Extraordinarily high densities of megafauna can be found near OMZ boundaries.
At the community level we see distinct taxonomic trends associated with OMZs. Among macrofauna, annelids dominate. Echinoderms and other heavily calcified taxa are often reduced within OMZs (Levin et al. 1991, 1997, 2000). Crustaceans and molluscs are less tolerant groups (Diaz and Roserberg, 1995), although there are certain taxa (Ampelisca, Astrys permodesta) that are exceptions (Levin, unpublished).
We might expect to see the same macrofaunal taxa distributed globally within OMZs. However, there is a surprising amount of variation. The top 4 species at stations with lowest oxygen in 4 regions of the world each are distinct (Table 1). Polychaetes dominate off Oman, where O2 concentration iss just above 0.1 ml L-1 . At 400 m on the Oman margin, most individuals belong to 2 species, a spionid Prionospio and a cirratulid, Aphelochaeta (Levin et al., 1997) Off Peru a single species of gutless, tubificid oligochaete (Phallodrilinae) comprises most of the macrofauna (Levin et al, in preparation. Surprisingly a gastropod (Astryris permodesta) is typically found near the sediment-water interface of the Santa Barbara Basin (Levin and Bernhard, unpublished). On Volcano 7, where the summit is covered by coarser foraminiferal sands, Aplacophorans and polychaetes dominate (Levin et al., 1991). Note that pogonophorans are now considered to fall within the Polychaeta. At the edge of the Santa Barbara Basin (555 m), oligochaetes also dominant, but there is a surprising diversity of taxa, including crustaceans, echinoderms and aplacophorans (Beaudreau, 1999). At the Basin center there are no macrofauna, although one meiofaunal polychaete appears in low numbers on a 0.3 mm screen.
Among the meiofauna, nematodes and calcareous foraminifera are most tolerant of low oxygen (Levin et al., 1991; Cook et al. 2000, Gooday et al., 2000, Neira et al., in press). This is illustrated by changing ratios of nematodes to harpacticoid copepods within the metazoan meiofauna. On the summit of Volcano 7 and on the Peru margin they are very high (500:1 and 65: 1, respectively) within the OMZ (Levin et al., 1991; Neira et al., in press). Beneath the OMZ, ratios are much lower. In studies of meiofauna off Oman, Peru and Mexico, harpacticoid copepods and agglutinated foraminifera are concluded to be especially intolerant to low oxygen (Gooday et al., 2000; Neira et al., in press; Levin et al., in preparation).
The taxonomic shifts described above are translated into changes in species diversity both with respect to dominance and species richness. Dominance of macrobenthos is extraordinarily high within OMZs . In a survey of 5 OMZ regions, the top ranked species comprises 47-87% of the total macrofauna (Table 1). Accompanying this high dominance is reduced species richness. Graphical representation of Rank 1 dominance and rarefaction measure of species richness (Es100) as a function of oxygen for bathyal sites around the world indicate that the effects of oxygen on diversity are evident only at oxygen levels below about 0.3 or 0.4 ml/l (. 2) Possible causes of reduced species richness within OMZs includes loss of species within taxa that are generally much less tolerant to low oxygen, for example the echinoderms, crustacean and molluscs . However, reductions in richness also occur within tolerant taxa such as the annelids (Levin and Gage, 1998). Organic enrichment may also contribute to reduced diversity, independent of oxygen. Separating the effects of hypoxia from those of organic enrichment within OMZs is difficult. Large scale, multiple regression studies by Levin and Gage (1998) suggest that within the polychaetes, oxygen exerts greatest control on species richness, while organic matter availability has more influence on measures of dominance and evenness. In the Arabian Sea one measure of food availability, sediment pigment concentration explained 70-90% of variation in indices of macrofaunal species richness, information index, dominance, and evenness (Levin et al. 2000).
Functional Processes
Trophic Pathways. One might expect that lying beneath the most productive waters in the world, the OMZ benthos would rely on heterotrophic consumption of this production. However, recent findings suggest that chemosynthesis plays an important role in OMZ systems in several ways (Levin et al. submitted). Numerous species possess endosymbiotic, sulfur oxidizing bacteria that fix and translocate carbon to the host or episymbiotic bacteria that may provide food as well. Still other species consume of freeliving bacteria or prey on species that do, or on species with symbionts. One of the most interesting examples of an OMZ species with chemoautotrophic endosymbionts is the gutless phallodrilinid oligochaete Olavius crassitunicatus, the dominant taxon at 300 m off Peru where oxygen concentrations are < 0.02 ml/l. This species possesses 3 types of subcuticular bacteria, at least one of which oxidizes sulfur (Giere and Krieger in press). This species forms 83% of the macrofauna present at this site and attains densities of over 13,500 individuals/m2 (Levin et al. in preparation). Other examples of symbiont-bearing taxa within OMZs include pogonophorans on Volcano 7, nerilid polychaetes and nematodes with episymbionts in the Santa Barbara Basin and lucinid clams on the Oman margin and in the Santa Barbara Basin. A recent paper by Bernhard et al. (2000) has shown symbioses to be the norm for protists and meiofaunal metazoans in the Santa Barbara Basin.
Bioturbation. Animal activities such as bioturbation and bioirrigation enhance oxygenation and solute transport, and speed the remineralization of organic matter. In general, bioturbation is reduced within OMZs (Savrda and Bottjer, 1991). Under extreme hypoxia or anoxia, all bioturbating organisms are absent and laminae or varves often form. Bioturbation of modern sediments has been quantified in 2 OMZ regions, on the Peru and Oman margins. In both cases the mixed layer depth, determined by Pb-210 and Th-234 profiles, is much lower within than beneath the OMZ (Smith et al. 2000; Levin et al., in preparation). On the Peru margin, particle mixing rates, measured by Th-234, are reduced within the OMZ (6-13 cm2/y) relative to station beneath the OMZ ( 80-150 cm2/y) (Levin et al. in preparation) No reduction in particle mixing rates was observed within versus beneath the Oman margin OMZ but a longer-lived tracer (Pb-210) was used (Smith et al. 2000).
Adaptations
to Permanent Hypoxia.
Adaptations of biota to OMZ conditions were reviewed by Childress and Siebel (1998), largely for planktonic organisms. These authors emphasize that animals living in OMZs must adapt to limited oxygen availability, not to a complete shortage. For even at very low oxygen concentrations there is enough oxygen available in the water if organisms can access it. Childress and Siebel (1998) proposed 3 general approaches that OMZ taxa can use to cope with low oxygen: (1) increased effectiveness of oxygen uptake (2) lower metabolic demands, and (3) use of anaerobic metabolism. They argue that the first of these is the most widely used approach. OMZ faunas do show lower metabolic oxygen requirements than shallow water relatives, but other deep-water species not living in OMZs do as well. The third approach is used mainly by vertically migrating plankton that can pay back oxygen debts incurred during daily migrations (up or down) to better- oxygenated water. In general all of these possible adaptations are poorly studied in benthic species.
Childress and Siebel (1998)
proposed five general methods by which organisms can t increase effectiveness
of oxygen uptake. Four
of these have been observed in OMZ benthic macrofauna.
High gill surface area is evident in Amepliscid amphipods, a group
that occurs in OMZs off Oman, Chile, Peru and California (Levin, unpublished
data). Elongate, proliferated and numerous branchiae appear to be adaptations
to permanent hypoxia in some spionid and dorvilleid polychaetes (Lamont
and Gage 2000, pers. obs). Cossurid polychaetes have exceptionally long
median antennae within the Oman margin OMZ (Lamont & Gage 2000). Increased gill surfacehas been documented in midwater mysids,
fishes,and cephalopods (Childress and Siebel 1998). Another possible adaptation, reduced diffusion distances, may
explain the success of small, thin, elongate taxa such as oligochaetes
and nematodes within OMZs.
Development of respiratory pigments (e.g., hemoglobins) with high
affinity for oxygen has been observed in benthic fish (Sebastolobus
alascanus) as well as pelagic fishes that live in the OMZ. Such adaptations
are likely in molluscs and other organisms where hemoglobins are observed.
(e.g., Amygdalum politum) (Levin, unpublished observations).
A variety of behavioral adaptions, including vertical migration
of plankters (Childress and Siebel 1998) and ontogenetic migrations (Wishner
et al. 2000) have been documented. Aplacophoran
molluscs within the OMZ on the summit of Volcano 7 seem to live with their
mantle permanently open, a possible adaptation to increase respiration
effectiveness (A. Scheltema pers. comm.).
High ventilatory ability and circulation capacity has been
documented in midwater crustaceans as a possible adaptation to OMZs but
has not been but studied in benthic species.
Remaining Questions
In general OMZ benthos are poorly studied and there exist more questions than answers about these systems. Key ecological questions that remain to be answered include the following:
1. What really controls standing stock in OMZs? Do oxygen and organic matter interact in determining abundances? What is the role of sulfides? Food cannot be the sole determinant of community structure because the most organic-rich system in the world off Peru has only small bodied organisms and low biomass (Levin et al., in preparation)
2. What are the physiological adaptations of benthic OMZ animals? Are there enzymatic adaptations? Do chemoautotrophic symbionts play a role?
3. What is the relative importance of chemosynthesis versus photosynthesis-based nutrition in OMZs?
4. What are the functional consequences of low diversity in OMZs? The effects of low diversity on ecosystem-level processes of production and remineralization are of considerable interest. OMZs with their low diversity may be a particularly good place to study these.
Concluding Remarks
Understanding the structure and function of modern OMZ faunas can help us to understand the past and possibly to predict the future. Modern OMZ faunas are considered analogs for construction of paleoecological low-oxygen models (Savrda and Bottjer, 1991). These include biofacies (body fossil), ichnofacies (trace fossil) and bioturbation models. These link the body or trace fossils of animals, and the amount of sediment mixing to the oxygen of overlying waters, or in some cases to productivity or organic matter availability. Scientist use this information to reconstruct the conditions in ancient seas. Studies of places such as the Peru margin, which are affected by interannual oxygen shifts, can help us understand system responses. For example, we have earned from studies of the Peru margin that bioturbation by non-feeding, symbiont-bearing forms can occur under almost anoxic conditions (Levin et al., in preparation)..
It is also likely that modern OMZs can provide clues about how a shallow water system might change should it move from episodic to permanent hypoxia. Expected changes in community structure and composition might be inferred. In addition the study of modern OMZs reveals the types of adaptations that animals might undergo or be selected for over ecological and evolutionary time.
Studies of OMZ benthos to date have been hampered largely by limited access to deep water systems in remote parts of the world. As scientists begin to understand the importance of these systems for nutrient cycling and for basic understanding of adaptations to extreme environments, our knowledge of these systems should increase tremendously.
Literature cited
Beaudreau,
A. 1999. Macrofaunal community responses to hypoxia in the Santa Barbara
Basin. UCSD SURF Program undergraduate report. 44 pp.
Bernhard,
J.M., Buck, K.R.., Farmer K.R., & Browser, S.S. (2000). The Santa
Barbara Basin is a symbiosis oasis. Nature,
403, 77-80.
Childress,
J.J. and B.A. Seibel. 1998. Life at stable low oxygen levels: adaptations
of animals to oceanic oxygen minimum layers. J. of Experimental Biology
201: 1223-1232.
Cook,
A.A, Lambshead, P.J., Hawkins, L.E., Mitchell, N. & Levin, L.A., 2000.
Nematode abundance at the Oxygen Minimum Zone in the Arabian Sea. Deep-Sea
Research. Part II 47: 75–85.
Diaz,
R.J. and R. Rosenberg. 1995. Marine benthic hypoxia: A review of its ecological
effects and the behavioral responses of benthic macrofauna. - Oceanogr.
Mar. Biol. Ann. Rev. 33: 245-303
Giere,
O., Krieger, J., In press, A
triple bacterial endosymbiosis in a gutless oligochaete (Annelida). Ultrastructural
and immunocytochemical evidence: Invertebrate Biology.
Gooday,
A. J., J. M. Bernhard, L.A. Levin, and S. Suhr. 2000. Foraminifera in
the Arabian Sea OMZ and other oxygen deficient settings: taxonomic composition,
diversity and relation to metazoan faunas. Deep-Sea Research 47: 25-54.
Jorgensen,
BB; Gallardo, VA. 1999. Thioploca
spp: filamentous sulfur bacteria with nitrate vacuoles. FEMS Microbiology
Ecology V28(N4):301-313.
Kamykowsky,
D. and S.J. Zentara. 1990. Hypoxia in the world ocean as recorded in the
historical data set. Deep-Sea Research 37(12): 1861-1874.
Lamont,
P.A. and J.D. Gage. 2000. Morphological responses of macrobenthic polychaetes
to low oxygen on the Oman continental slope, NW Arabian Sea. Deep-Sea
Research 47: 9-24.
Levin,
L.A. and J.D. Gage. 1998. Relationships
between oxygen, organic matter and the diversity of bathyal macrofauna.
Deep-Sea Research. 45: 129-163.
Levin,
L.A., J.D. Gage, P. Lamont, L. Cammidge, C. Martin, A. Patience, and J.
Crooks. 1997. Infaunal community structure in a low-oxygen, organic-rich
habitat on the Oman continental slope, NW Arabian Sea. pp. 223-230 In:
L.E. Hawkins and S. Hutchinson (eds). The Responses Of Marine Organisms
to Their Environment. Univ. of Southampton.
Levin,
L.A., J. D. Gage, C. Martin, P. Lamont. 2000. Macrobenthic community structure
associated with the oxygen minimum zone, NW Arabian Sea: Pattern and Cause.
Deep-Sea Research 47: 189-226.
Levin,
L.A., Gallardo, V.A. and R. Michener. Submitted. Chemosynthesis supports
oxygen minimum zone animal communities. Natur.
Levin,
L.A., Gutiérrez, G., Rathburn, A., Neira, C. Sellanes, J., Munoz,
P., Gallardo, V., Salamanca, M. and Schuffert, J. in preparation.
Benthic processes on the Peru Margin:
A transect across the oxygen minimum zone during the 1997-98 El
Niño. For Progress in Oceanography
Levin,
L.A., C.L. Huggett and K.F. Wishner. 1991a. Control of deep-sea benthic
community structure by oxygen and organic-matter gradients in the eastern
Pacific Ocean. Journal of Marine Research 49: 763-800.
Neira,
C, Sellanes, L. Levin, L.A. and Arntz, W.E. in press. Meiofaunal distributions
on the Peru margin: relation to oxygen and organic matter availability.
Deep-SeaResearch.
Rogers 2000 DSR
Sanders,
H. 1969. Benthic marine diversity and the Stability-Time Hypothesis. Brookhaven
Symposium in Biology. 22: 71-81.
Savrda,
C.E. and D.J. Bottjer. 1991. Oxygen-related biofacies in marine strata:
an overview and update. In Tyson, R.V. and T.H. Pearson (eds.),
Modern and Ancient Continental Shelf Anoxia, pp. 201-219, Geological Society,
Special Publication 58.
Smith,
C.R., L.A. Levin, D.J. Hoover, M. Cremer, G. McMurtry,
and J.D. Gage. 2000. Bioturbation across the oxygen minimum zone
on the Arabian-Sea Slope. For DSR Special Volume 47: 227-257.
Tarazona, J., H. Salzwedel & W. Arntz, 1988. Positive
effects of “El Niño“ on macrobenthos inhabiting hypoxic areas
of the Peruvian upwelling system. Oecologia 76: 184–190.
Thompson,
J.B., H. T. Mullins, C.R. Newton and T. Vercoutere. 1985. Alternative
biofacies model for dysaerobic communities. Lethaia 18: 167-179.
Tyson,
R.V. & T.H. Pearson, 1991. Modern and ancient continental shelf anoxia:
an overview. In R.V. Tyson & T.H. Pearson, Modern and ancient continental
shelf anoxia. Geological Society Special Publication No. 58, London, 1–24.
Wishner,
K., C. J. Ashjian, C. Gelfman, M. Gowing, L. Kann, L. Levin, L. Mullineaux,
and J. Saltzman. 1995. Pelagic and benthic ecology of the lower interface of the Eastern
Tropical Pacific oxygen minimum zone.
Deep-sea Res., 42: 93-115.
Wishner,
KF; Gowing, MM; Gelfman, C. 2000
Living in suboxia: Ecology of an Arabian Sea oxygen minimum zone copepod.
Limnol. Oceanogr. 45: 1576-1593.
Wishner,
K., L. Levin, M. Gowing, and L. Mullineaux. 1990. Involvement of the oxygen
minimum in benthic zonation on a deep sea mount. Nature 346: 57-59.
Wyrtki,
K., 1966. Oceanography of the eastern Pacific Ocean. Oceanogr.
Mar. Biol. Ann. Rev.
4: 33–68.
Wyrtki,
K. 1973. Physical oceanography of the Indian Ocean. In Zeitzschel,
B. (Ed.). The Biology of the Indian Ocean, pp. 18-36, Springer-Verlag,
Berlin.
Figures and Tables:
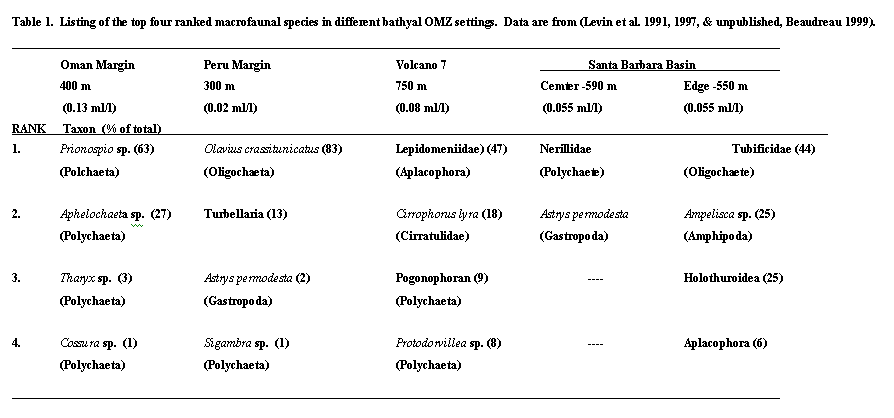
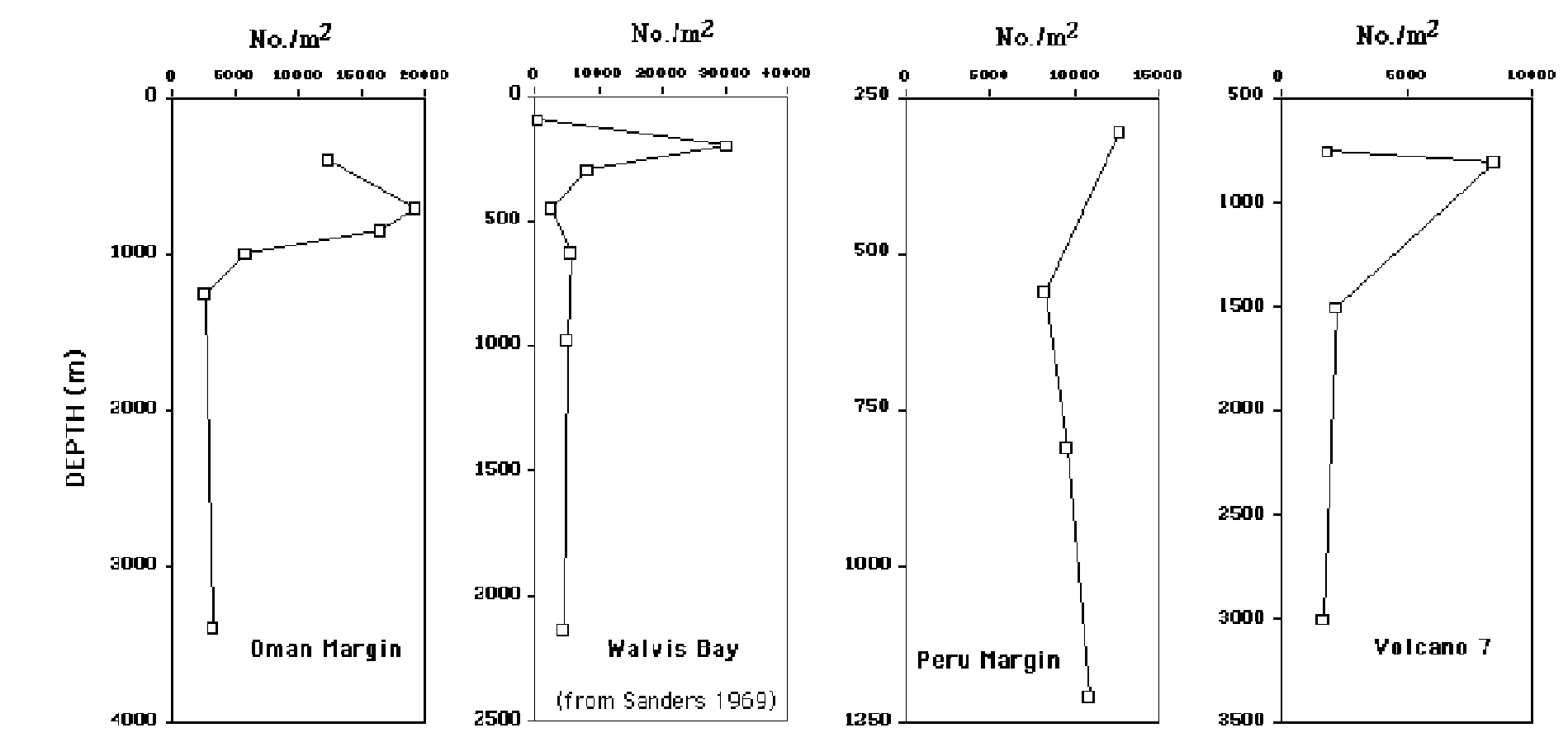
Figure 2. Average density of macrofauna (> 300 microns) at bathyal stations along transects through oxygen minima. Note distinct maximum at 3 of 4 locations. Data are from Sanders (1969), Levin et al. (1991), Levin et al. (2000) and Levin et al. (submitted).
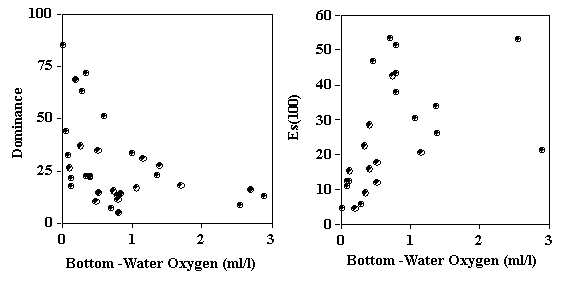
Figure 3. Plot of Rank 1 Dominance and Expected Species Richness (ES100) as a function of bottom-water oxygen concentration for bathyal macrofauna from the eastern Pacific and northern Indian Oceans.